Research
Overview
In Sugahara laboratory, we make full use of the synthetic techniques of inorganic synthetic chemistry in seamless combination with those of organic, organometallic and polymer chemistry mainly to develop the preparation methods for organic-inorganic hybrid materials and ceramic materials.
Our current main research topics are as follows:
- We work on the preparation and surface modification of inorganic nanomaterials to form so-called element blocks, hybrid nanomaterials. We further disperse them in polymer matrices to obtain bulk organic-inorganic hybrid materials. We place special emphasis on establishment of techniques for surface modification of metal oxide nanomaterials using organophosphorus compounds (typically organophosphonic acids and phosphoric acid esters) as coupling agents.
- We prepare ceramic materials by using inorganic polymers as precursors via pyrolysis. By combining precursor preparation techniques with other techniques such as high-pressure synthesis, we aim at exploring the preparation of new functional materials and developing new preparation methods for materials.
- We develop preparation methods for organic-inorganic hybrid materials with built-in functional units by connecting element block molecules containing heteroatoms. We utilize inorganic network formation processes such as sol-gel reactions as well as living radical polymerization reactions utilized in polymer chemistry.
Recent research
Preparation of functional nanosheets and their uses for improving the physical properties of epoxy resin
Improvement of thermal stability and water resistance have been desired for epoxy resin. Thus, organic-inorganic hybrid materials were prepared by dispersing layered-perovskite-derived nanosheets covered with hydrophobic fluoroalkoxy groups in epoxy resin. Dispersal of such nanosheets in epoxy resin led to improvement of their thermal stability and suppression of their water adsorption behavior.
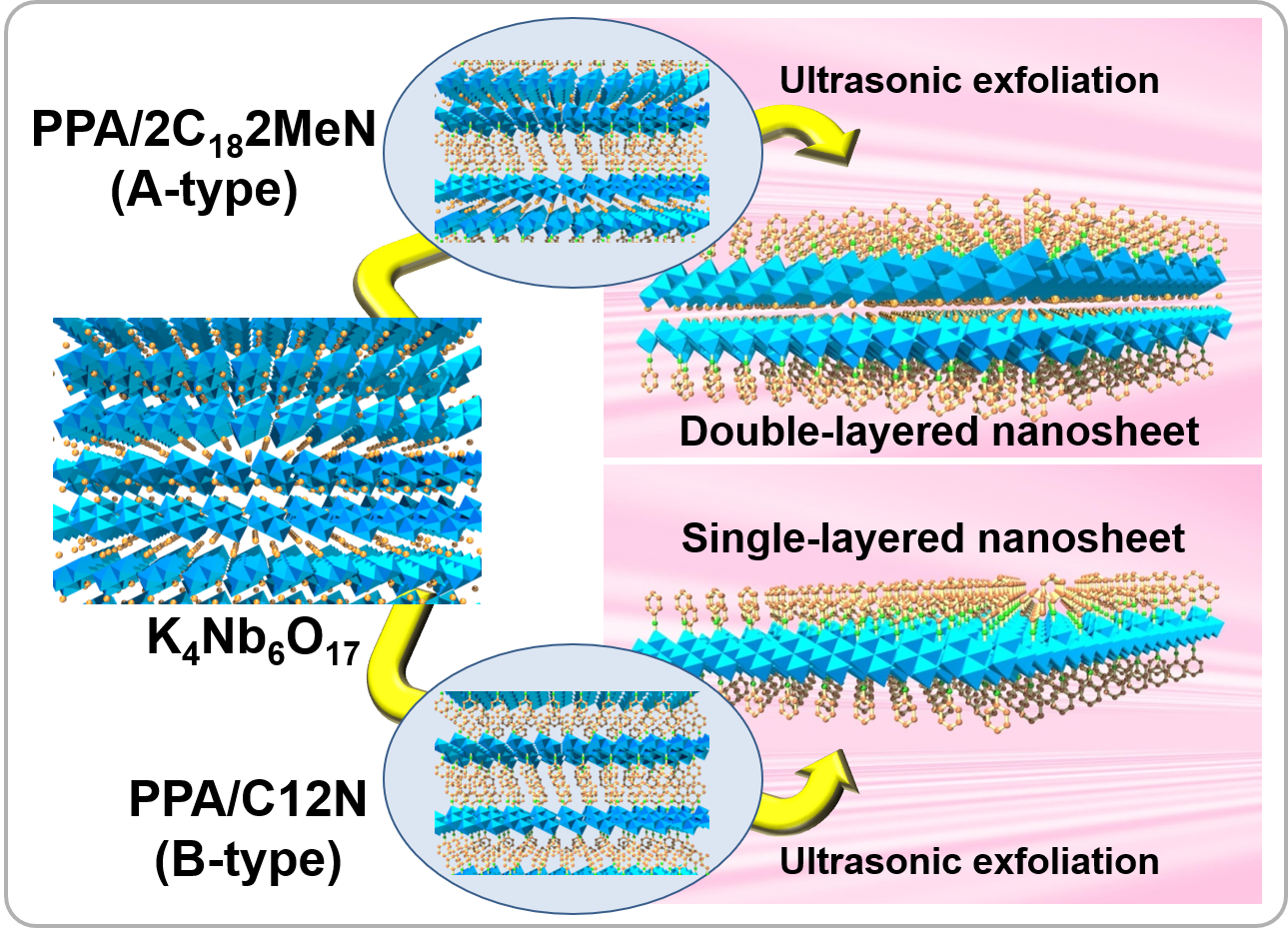
Preparation of single- and double-layered nanosheets
With previous preparation techniques for nanosheets, it was difficult to prepare nanosheets composed of multiple layers. Potassium hexaniobate exhibits a unique structure, however, in which two kinds of interlayers (interlayersⅠ and Ⅱ) appear alternately. We used their different reactivities to achieve selective surface modification of interlayerⅠand surface modification of both interlayers Ⅰ and Ⅱ using phenylphosphonic acid. Upon exfoliation of these layered organic derivatives, preparation of double- and single-layered nanosheets was successfully achieved.
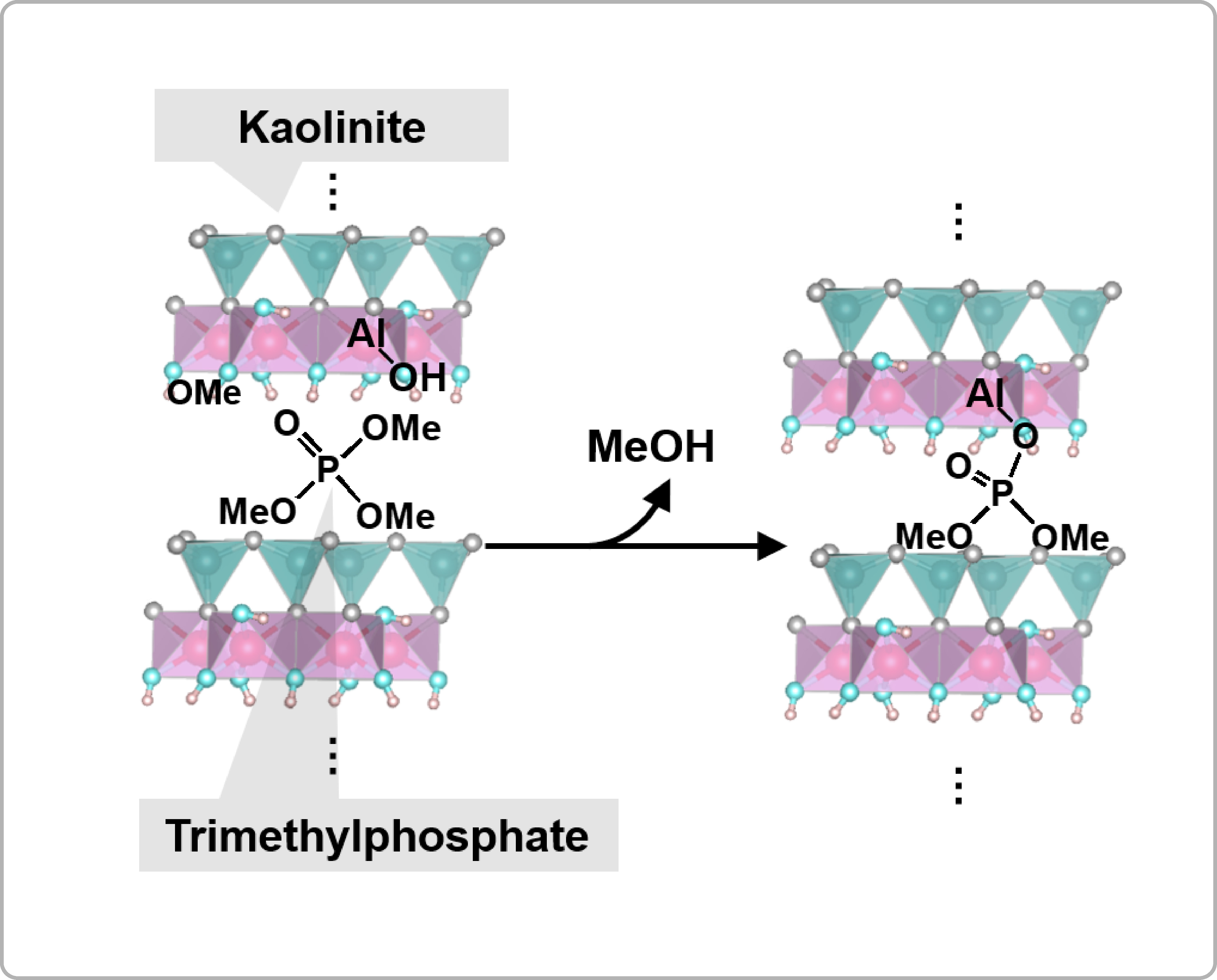
Successful surface modification of layered clay minerals bearing hydroxyl groups using phosphoric acid triesters (PO(OR)3)
The above-mentioned surface modification using organophosphonic acids could exhibit disadvantages for some layered compounds: the strong acidity of POH groups in phosphonic acids could destroy some inorganic surfaces. Kaolinite, a unique layered clay mineral bearing interlayer hydroxyl groups (Al2Si2O5(OH)4), has been widely used since ancient times as a raw material for pottery. The attempt to modify kaolinite surfaces with an organophosphonic acid failed, however, due to decomposition of the kaolinite structure. By contrast, we achieved the modification of kaolinite surfaces using trimethlyphosphate, the smallest phosphoric acid triester.
Preparation of titanium dioxide nanoparticle-containing polymers with a high reflective indices
Although the need for polymer materials with high reflective indices has increased, common polymeric materials exhibit relatively low reflective indices. Thus, preparation of organic-inorganic hybrid materials with high reflective indices has been achieved by many researchers by incorporating titanium dioxide with a high reflective indeices into polymer matrices. We have successfully prepared hybrid materials with high reflective indeices and transparency via surface modification of titania nanoparticles with phosphorus coupling agents and achieved high dispersibility with no obvious aggregation.
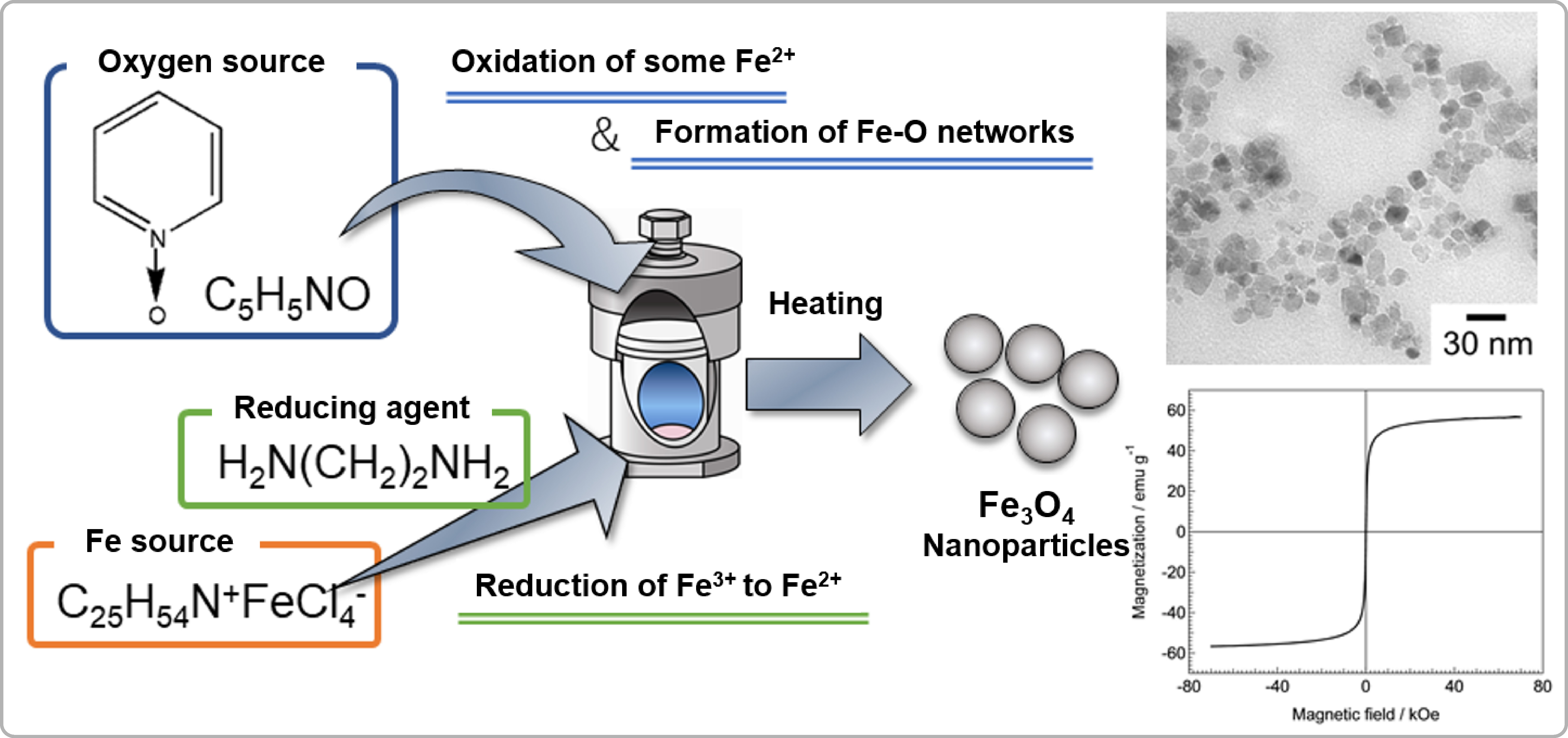
Preparation of magnetite nanoparticles via a nonaqueous process with control of the amount of an oxygen donor
Transition metal oxide nanoparticles (NPs) exhibit various attractive physical properties. Among transition metal oxide NPs, magnetite (Fe3O4) NPs exhibit interesting magnetic properties, superparamagnetism, if their particle sizes are appropriate. Thus, control of their particle sizes is desirable. We succeeded in controlling the particle sizes by using organic oxygen donors to limit the amount of oxygen supplied. Combining a non-aqueous process with control of the amount of the oxygen donor, pyridine-N oxide, fine superparamagnetic Fe3O4 NPs were successfully prepared.
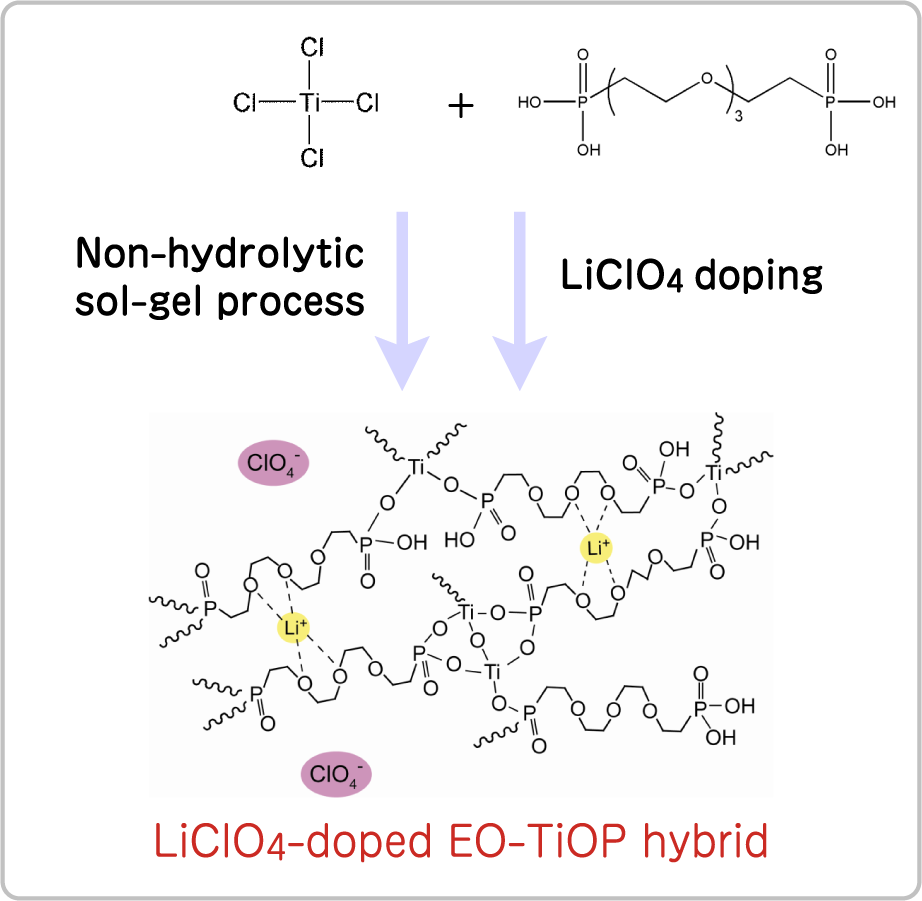
Preparation of a Li-ion conductor using diphosphonic acid-bearing oxyethylene chains
Although Li-doped poly(ethylene oxide) could be an excellent Li-ion conductor, its crystallization decrease ion to significantly conductivity proceeds at room temperature. Thus, organic-inorganic hybrid materials were prepared through a reaction of diphosphonic acid bearing a short oxyethylene chain with tetratitanium chloride and subsequent Li ion doping. The ionic conductivity values of the resultant organic-inorganic hybrid materials were on the order of 10-5 S cm-1 which are relatively large values despite containing a short oxyethylene chain. In addition, there was no obvious decrease in ionic conductivity corresponding to the crystallization of these hybrid materials.
Preparation of cubic-phase silicon nitride using amorphous materials as precursors
Polymer derived ceramics (PDCs) have been developed for preparation of non-oxide and oxide ceramics from preceramic inorganic polymers. Cubic silicon nitride, a recently discovered superhard materials, is generally prepared by high-temperature, and high-pressure treatment of α- and β-type silicon nitrides. In this study, cubic silicon nitride was prepared by high-temperature, and high-pressure treatment of pyrolysis products obtained by thermal decomposition of polysilazane, an inorganic polymer with a Si-N backbone, at relatively low temperature. Changing the pyrolysis temperature to control crystallization of pyrolysis products revealed that the use of amorphous pyrolysis products was advantageous for preparation of cubic-phase silicon nitride.